Features Brief
Veeva Study Training
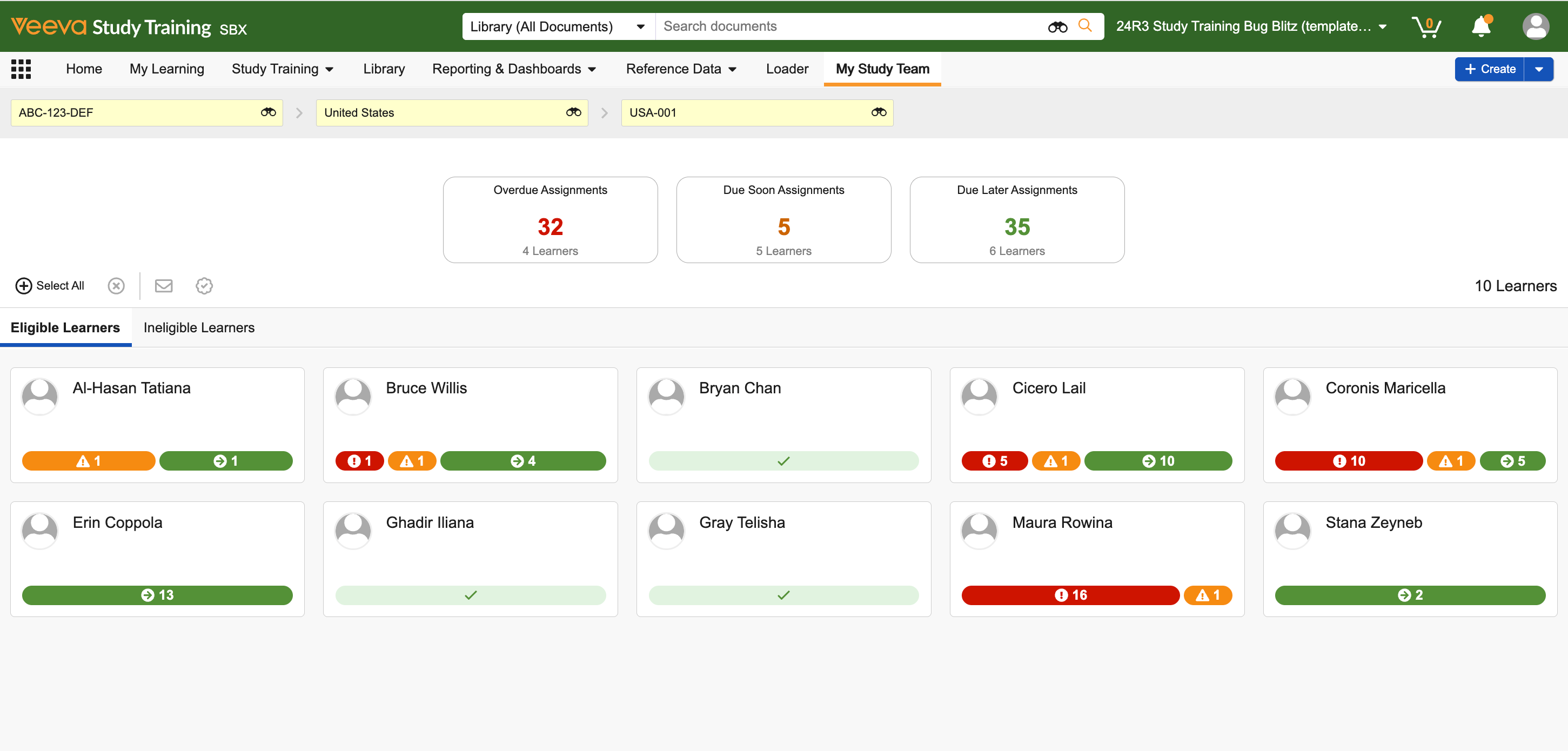
Train Sponsors, CROs, and Sites in One Application
Clinical studies are reaching new levels of complexity and scale, with an average study now requiring hundreds of personnel spread across multiple countries. Using disparate systems to manage study training across large, dispersed teams over the course of a study duplicates efforts, reduces visibility, and increases compliance risk. Ensure inspection readiness by eliminating manual trackers and generating inspection-ready training certificates.
Veeva Study Training brings sponsors, CROs, and research sites into a single platform to streamline and automate training while ensuring inspection readiness. With connectivity to Veeva Clinical Operations, Study Training improves training creation, assignment, and delivery. Pre-configured reports and automated alerts for new and updated curricula drive greater visibility, oversight, and compliance.
Business Benefits
- Streamline and automate study training by bringing sponsors, CROs, and research sites into a single platform.
- Ensure inspection readiness by eliminating manual trackers and automatically filing inspection-ready training certificates in Veeva eTMF.
- Improve compliance with automated training alerts and on-demand curricula access for study personnel.
- Improve oversight with unified reporting across sponsors, CROs, and sites.
Features
-
Single Platform for All Study Personnel
Manage study training curricula for sponsors, CROs, and research sites in one streamlined platform. -
Seamless Connection with Veeva Clinical Operations
Leverage clinical master data and version-controlled documents from Veeva Clinical Operations to automate study training, and auto-file evidence of training completion in Veeva eTMF. Veeva Site Connect can also be leveraged to drive task-based training in Veeva Study Training. -
Digital Matrix for Training Assignments
Quickly assemble a training matrix for each study and assign training by study team role and individual responsibilities. -
Automated Training Assignment and Distribution
Use automated alerts for new or updated curricula to ensure study and site teams stay current with training, including protocol training. -
Cross-Sponsor GCP Credit Transfer
Enable site staff to submit proof of GCP training completion from other sponsor Vaults, reducing overtraining. -
Quizzes and Assessments
Ensure competency with flexible, easy-to-configure quizzes that provide immediate feedback to remediate incorrect answers. -
Unified Reports and Dashboards
Monitor oversight and compliance in real-time with pre-built dashboards and reports, or build your own. -
Compliance and Validation
Drive compliance with audit trails, e-signatures, validation processes, and configurable business logic designed in a strict change control environment.
About Veeva Clinical Operations
Veeva Clinical Operations empowers clinical teams with a unified platform for efficient trial execution. Streamlining processes and improving data visibility from start-up through closeout accelerates timelines and enhances collaboration across sponsors, sites, and CROs.
Learn how you can streamline and automate study training while ensuring constant inspection readiness by visiting veeva.com/VeevaStudyTraining.